5. DETERMINATION OF PHENOLS
Phenols react with the alkali hydroxides,
giving rise to water-soluble phenolates. This is the basis of the classical
method98 for the estimation of phenols in essential oils. Since the potassium
salts of many phenols are more soluble than the corresponding sodium salts, the
use of potassium hydroxide is preferred.
It must be remembered that, in addition to
the phenols, any alkalisoluble material (e.g., acids) will also go into
solution as well as any watersoluble constituents or water-soluble adulterants
(e.g., alcohol). This will give rise to erroneous results: the apparent phenol
content will be too high.
Further, an aqueous solution of alkali
phenolates is a much better solvent for the nonphenolic portion of an oil than
is the alkali solution itself; specifically this is important in the case of
terpeneless bay oils.
When the determination has been completed, it
often proves of value to separate the nonphenolic portion and to study its
odor. The alkaline solution of the phenolates may be freed of traces of oil by
washing with ether; the phenols may then be regenerated by the addition of
dilute sulfuric acid (1:3), extracted with ether, and obtained in a pure state
by evaporating off the ether. (The separated ether layer should be dried with
anhydrous sodium sulfate before this final evaporation.) The presence of
foreign phenolic bodies frequently may be detected by this technique.
Modifications of the general procedure become
necessary in the case of certain specific oils. Such modifications are noted
below.
GeneralProcedure:
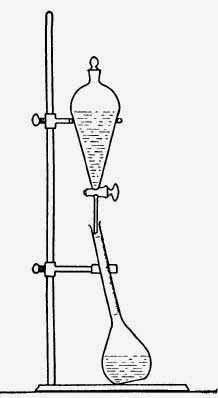
Into a well
cleaned 150 cc. cassia flask, having a long, thin neck graduated in 0.1 cc.
divisions, introduce 10 cc. of the oil, measured from a pipette. Add 75 cc. of
an aqueous 1 N potassium hydroxide solution," measured from a graduated cylinder.
Stopper and shake thoroughly for exactly 5 min. Permit to stand undisturbed for
1 hr., after which the undissolved oil is forced into the neck by the addition of
more potassium hydroxide solution. The alkaline solution must be added
carefully to avoid disturbing the layer of separated oil. (This addition may
conveniently be made by clamping the flask at a slight angle on a ring stand;
above the flask is placed a ring to hold a separatory funnel containing the
solution of alkali which is permitted to flow down along the inside of the neck
of the cassia flask very slowly. If the flow of the alkali is adjusted to about
1 drop per sec., a clean separation of the oil is usually obtained. (See
Diagram 4.10.) In order to make any droplets of oil adhering to the sides of
the flask rise into the neck, gently tap or revolve the flask rapidly between
the palms of the hands. Measure the quantity of oil that does not dissolve in
the alkali. The phenol content, expressed as a volume/ volume percentage, is
calculated from the following formula :
Percentage of phenol = 10(10 no. of cc. of
undissolved oil)
DIAGRAM 4.10. Apparatus for Phenol determination.
Oils containing large amounts of heavy metals
may not give a sharp separation of the nonphenolic oily layer and the alkaline
solution in the neck of the flask. Such oils should be thoroughly shaken with a
small amount (about 1 per cent) of powdered tartaric acid and filtered to
remove the interfering metals before the determination of phenols is attempted.
Modification of the General Procedure:
I. Clove Oils.
Since clove oils
contain aceteugenol in addition to free eugenol and since both constituents
contribute to the value of the oil it is customary to saponify the former and report
the total phenol content as eugenol. The general procedure is modified as
follows :
After thoroughly shaking the oil and alkali
for 5 min. in the cold, heat the flask on a steam bath for 10 min. Occasionally
shake the flask during this heating to insure complete saponification. Immediately
after removal of the flask from the steam bath add a further quantity of alkali
in order to drive the unreacted oil into the neck of the flask. It is necessary
to make this addition while the content of the flask is still hot since the nonphenolic
portion may partially solidify.
II. Pimento Oils.
The procedure
described above for clove oils is also used for the determination of the phenol
content of pimenta oils.
III. Terpeneless Bay Oils.
Because of the solvent effect of the potassium eugenolate upon the nonphenolic
constituents of a terpeneless bay oil, the whole oil will go completely into
solution if a 1 N solution of potassium hydroxide is used in this determination.
Therefore, it becomes necessary to reduce the strength of the alkali to 3% and
to use 125 cc. of this dilute alkaline solution for shaking out the phenols.
IV. Cinnamon Oils.
These oils
offer some difficulty to theanalyst. The formation of a troublesome emulsion
and a very poor separation of the oil and the aqueous layers results because of
the similarity of the gravity of the oil and the gravity of the solution. It is
for this reason that a 3% solution cannot satisfactorily be used. Shaking for
too long a period gives rise to results that are much too high. The following
procedure, if followed exactly, will give results that can easily be
duplicated, and which represent approximately the true eugenol content:
To 50 cc. of a 1 N potassium hydroxide
solution in a cassia flask add 5 cc. of the cinnamon oil. Shake well for
exactly 3 min. and let stand for 10 min. Fill the flask with potassium hydroxide
solution, using the ring stand technique described under the general procedure.
If the determination has been carried out carefully, the residual oil will rise
into the neck in an unbroken column.
V. Thyme and Origanum Oils.
The
phenolic constituents of thyme and origanum oils consists mainly of thymol and carvacrol.
The separation of the phenolic constituents is an aid in the evaluation of
these oils, since oils containing predominently thymol are generally considered
of superior quality. Thymol is easily crystallized ; carvacrol is a liquid at
temperatures above 2.
The separation and examination of the
phenolic portion may conveniently be carried out after the determination of the
phenol content.
Pour the contents of the cassia flask (used
in the assay) into a separatory funnel and permit the nonphenolic portion to separate.
Filter the aqueous layer through filter paper previously wetted with water.
Transfer this filtered solution to a separatory funnel and acidify with dilute
hydrochloric acid (1:3) until the mixture is strongly acid to litmus. Add 50
cc. of ether and shake thoroughly. Separate the ether layer, dry with anhydrous
sodium sulfate and filter. Evaporate the ether cautiously on a steam bath and
pour the liberated phenols into a test tube, and permit it to stand at room
temperature for 30 min. If the phenols consist primarily of thymol, a
crystalline mass results. If no crystals form after 30 min., cool to 5 by means
of an ice bath. Rub the side of the test tube with a thermometer or glass rod
and add a small crystal of thymol to initiate crystallization: if no crystals
form after 30 min. the absence of an appreciable amount of thymol may be
assumed.
In determining phenol contents it is well to
remember that watersoluble constituents may be added to increase the apparent
phenol contents. Alcohol and certain glycols are such adulterants which may be
occasionally encountered. If the relationship between the specific gravity and
the phenol content appears abnormal, the oil should be investigated further for
the presence of possible adulterants.
---------------------------------
98 The procedure was first applied by Gildemeister for
the determination of phenols in thyme oil.
99 Gildemeister and Hoffmann, "Die atherischen Ole,"
3d Ed., Vol. I, 753, recommended the use of a 5 per cent solution of either NaOH
or KOH be employed for thymol and carvacrol-containing oils ; a 3 per cent solution
for eugenol-containing oils. The official methods of the "United States Pharmacopoeia"
and the "National Formulary" require the use of KOH T.S. solution (1 N).


1 Comment:
This is amazing thanks for sharing this blog I become fan of your blogs now. This blog is so interesting and informative.
Post a Comment