2.2 Sesquiterpenoids
The sesquiterpenoids are
found to be extensively distributed in nature and by all means represent the
most abundantly prevailing class of terpenoids. A few typical examples
are cited below:
Sesquiterpenoids may be classified into four
major categories namely: acyclic, monocyclic, bicyclic and tricyclic
sesquiterpenoids, as summarized in Table 5.1.
Sesquiterpenoid Lactones Interestingly,
another class of compounds essentially bearing such characteristic features as
an α-methylene γ-lactone system; α, β-unsaturated
carbonyls, and epoxides and obiviously chemically distinct from the sesquiterpenoids
are collectively termed as sesquiterpenoid lactones. The specific
and vital biological nucleophilies e.g.; thiol and amino moieties
present in the enzymes, help in the augmentation of faster and reactive
approach to receptor sites by these sesquiterpenoid lactones. Thus, the
overall effect is evidenced by a marked and pronounced biological activities,
for instance: modified antimicrobial activity, enhanced antitumour properties.
Table 5.1 Classification of
Sesquiterpenoids with Summarized Details
In general, the sesquiterpenoid lactones are
classified into three major group as summarized below:
It would be worthwhile to mention some typical
examples of natural products that have the sesquiterpenoid lactone functions,
namely: artemisinin.
2.2.1 Artemisinin
Biological Source It is obtained from the leaves and the closed, unexpanded flower heads of Artemisia annuna Linn., belonging to family Asteraceae.This particular herb has been used in the Chinese system of medicine exclusively for the treatment of malaria since more than one thousand years.
Geographical Source The plant grows abundantly in China.
Chemical Structure Though the herb was used for its wonderful proven therapeutic efficacy for more than a decade centuries, but its active principal artemisinin was isolated and identified in 1972.
It has been established experimentally that the presence of an internal peroxide linkage
strategically located in the seven membered ring is an absolute necessity for it to exert the unique antimalarial property.
Modifications in Structure On account of the poor water solubility of artemisinin an attempt was made to improve either its water solubility ir its lipid solubility. In the former instance, Sodiumartesuna te i.e., the sodium salt of its hemisuccinate ester was developed; while in the latter instance, Artemether i.e., its corresponding methyl ether analogue was produced. Evidently, sodium artesunate is employed for intraveneous injections and artemether is used as a potent long acting drug.
Uses
1. The drug and its derivatives are used as fast acting blood schizontocides in the control and management of malarial fever caused by plasmodium vivax strain.
2. These drugs are found to be active against both chloroquine resistant and chloroquine sensitive strains of Plasmodium falciparum.
3. These drugs are found to show extremely encouraging therapeutic effects specifically in the treatment of Cerebral malaria by virtue of their significant rapid clearance of the prevailing parasites when compared to either chloroquine or quinine (synthetic antimalarials)
Note: The chances of recurrence is quite substantial by the treatment of artemisinin and its derivatives; therefore, it is always necessary to adopt the course of a combination therapy employing other antimalarials.
2.2.2 Parthenolide
Biological Source It is obtained from the leaves of Tanacetum parthenium (L.) Schultz-Bip, belonging to family Asteraceae. It is commonly known as feverfew and has been employed for centuries as an effective febrifuge (antipyretic) which perhaps suggested the original nomenclature.
It is also obtained from Chrysanthemum parthenium (L.) Bernh. Family Compositae;and
Magnolia grandiflora (L.) family Magnoliaceae.
Geographical Source The plant M. grandiflora is a native of North America and also cultivated in Indian gardens.
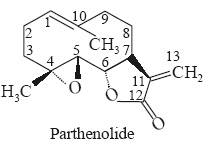
Chemical Structure Parthenolide is a sesquiterpenoid lactone having the following structure with the chemical name 4,5α-epoxy-6 β-hydroxy germacra-1 (10), 11(13)-dien-12-oic acid γ-lactone.
It has an additional epoxide bridge between 4-and 5α-positions.
Uses
1. It is found to act as a serotonin antagonist thereby causing an inhibition of the release of serotonin from blood platelets.
2. Based on the findings conducted by an elaborated double blind placebo-controlled clinical trials have established that the drug is significantly effective in the prophylaxis of migraine by reducing considerably the severity as well as the frequency of the pain due to headache.
3. A normal dose of 125 mg per day of good quality dried leaves either in the form of tablets or
capsules are used in the therapeutic practice as an antipyretic or febrifuge.
2.2.3 Matricarin
Biological Source It is obtained from the dried flower heads of Matricaria chamomillaL., and Artemnisia tilesii Ledeb, belonging to family Compositae. It is also found inMatricaria recutita Linne., family Asteraceae which represent the drug usually termed asGerman Chamomile. Besides, an allied plant source Chamaemelum nobilc Linne, normally known as Roman Chamomile also comprises of identical components and used alike.
Geographical Source In general, the above two chamomiles are cultivated abundantly in various parts of Europe.
Chemical Structure Its chemical name is 8α-acetoxy-4α-hydroxyguaia-1(10), 2-dien-12, 6αolide.
Uses
1. Chamomile has acclaimed to be the most popular ‘herbal tea’ in the United States because of its definite anti-inflammatory and antipasmodic therapeutic properties.
2. The volatile oil of M. chamomilla contains the sesquiterpenoid (-)-α-Bisabolol (Bisabolane) which exerts anti-inflammatory activity.
3. An infusin (tea) when consumed over a long span results into a cumulative overall positive effect which certainly justifies its age-old usage as an unique home-remedy and healthy beverage not only in Europe but also in the United States.
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar








0 Comment:
Post a Comment