2.6.5.3.1 Aliphatic Terpene Aldehydes
The two important members of this particular class are namely, citral and citronellal.
A. Citral
Chemical Structure 3,7-Dimetyl-2,6-octadienal; (C10H16O); Citral from natural sources is a mixture of two geometric isomers Geranial and Neral.
Occurrence It occurs abundantly in the oil of lemon grass (75 to 85%) [Cymbopogan flexuosus (Ness) stapf. And Cymbopogon citratus (DC) stapf. Family : Graminae]. It is also present to a limited extent in oils of verbena, lemon, lime, orange and ginger root. It is reported to be present in various other species, namely: Ocimum pilosum (35%), Liptospermum citratum, Eucalyptus staigeriana and in the leaf oils of several Citrus species etc.
Isolation The rich citral containing volatile oils e.g., lemon grass oil is thoroughly shaken with 5%(w/v) sodium bisulphite solution for about 25-30 minutes. The resulting crystalline adduct is first separated on a Büchner funnel, and subsequently washed with solvent ether or ethanol to remove the impurities. The crude citral is usually regenerated by decomposing the sodium bisulphite adduct with dilute sodium hydroxide solution carefully. Finally, the pure citral is obtained by distilling the crude citral cautiously under reduced presure (bp26 92–93oC).
Separation of Geranial (Citral-a) and Neral (Citral-b) Tiemann* observed that geranial may be obtained free from neral during the process of regeneration from the bisulphate adduct, by taking the strategic advantage of the fact that the crystalline sodium bisulphite adduct of geranial is sparingly soluble, whereas the corresponding adduct of neral is readily soluble in water. Tiemann** further observed that neral may be isolated from the regular citral (mixture) by shaking it for a short time with alkaline cyanoacetic acid solution (NC.CH2.COOH), when geranial reacts with this acid much faster than neral. Thus, we may have:
-------------------------------------------------
* Tiemann, Semuler, Ber. 26, 2708 (1893).
** Tiemann, Semular, Ber. 31, 3310, 3317 (1898).
Linalool
Identification
1. By virtue of the presence of two ethylenic and one aldehydic linkage citral is very sensitive to oxidizing agents (even exposure to air) to yield linalool having an intensified yellow colour.
2. Geranial on treatment with ammoniacal silver nitrate (Tollen’s Reagent) gives rise to geranic acid (C19H15COOH).
3. Hydrogenation of geranial with sodium amalgum in faintly acidic solution yields citronellal and citronellol.
4. Treatment with potassium bisulphate or diluted sulphuric acid geranial gets converted to paracymene with the loss of a molecule of water.
5. Citral when digested with acetic anhydride and sodium acetate gives rise to its enolic form as given below:
6. Syntheses of Pseudo- and α and β-Ionones: Citral undergoes condensation with substances containing a reactive methylene group as depicted below in sections (a) and (b) respectively:
(a)
Interaction of acetone and citral gives rise to the formation of pseudo-ionone (or ψ-ionone) with the loss of water molecule.
In general, the aliphatic ketone pseudo ionone undergoes cyclization with the aid of a variety of reagents, namely: sodium acetate, conc. sulphuric acid, formic acid, dilute mineral acids, sodium bidulphate etc., as stated above.
7. Citral may also be identified by the preparation of derivatives such as:
(i) the 2,4-dinitrophenyl hydrazones: Citral-a mp 108-110°C; and Citral-b mp 96°C;
(ii) the semicarbazones: Citral–a mp 164°C and Citral-b mp 171°C.
Uses
1. It is used extensively in the synthesis of vitamin A, ionone and methylionone.
2. It is employed as a flavour for fortifying lemon oil.
3. It is used widely in perfumery for its distinct citrus effect in lemon and verbena scents , in cologne odours and in perfumes for coloured toilet soaps.
B. Citronellal
Chemical Structure 3,7-Dimethyl-6-octenal; (C10H18O);
Occurrence It is the chief constituent of citronella oil (Cymbopogon winterianus and Cymbopogon nardus, family: Poaceae). It is also found in a variety of volatile oils, for instacne: lemon, lemon grass, melissa* (Melissa officinalis, fam: Lapiatae) and rose. Due to the presence of one asymetric C-atom in citronellal it can exist in racemic (dl-) form , d- and l-forms. However, the d-form occurs as the chief constituent in the oil of citronella obtained from Eucalyptus citriodora and other species of Eucalyptus (fam: Myrtaceae) whereas the l-form occurs exclusively in Java Lemon Oil.
----------------------------------------
* Spoon, Chem. Weekbl., 54, 236 (1958).
Isolation It may be conveniently isolated from essential oils by the formation of its crystalline bisulphite adduct. Interestingly, citronellal esentially possesses an ethylenic and an aldehyde moiety by virtue of which three different bisulphite adducts are possible theoretically as given below:
Note These three structural analogues have been prepared actually under various experimental parameters. However, the complete decomposition and subsequent regeneration of the desired aldehyde (i.e. Citronellal) may be accomplished easily by treating the ‘normal bisulphite adduct’ either with dilute mineral acids or with alkali carbonates. Strong alkalies eg., NaOH and KOH must be avoided so as to cause resinification of the aldehyde. Separation of Citronellal from Citral There are two separate procedures adopted for the separation of citronellal from citral as discussed here under:
(a) Tiemann’s Method*: It is based on the fact that citronellal reacts exclusively with a
concentrated solution of sodium sulphite and sodium bicarbonate, whereas citral reacts even with a dilute solution.
(b) Gildemeister Hoffmann’s Method**: It is solely guided by the fact that with neutral sodium sulphite, citronellal yields hydrosulphonic derivatives from which the latter cannot be recovered. However, the reaction shall commence only if:
(i) right from the beginning a strong current of pure CO2 is made to pass through the
reaction mixture, or
(ii) another acid is added gradually to the reaction mixture in sufficient quantities.
The said reaction of citronellal with neutral sulphite may afford its separation from citral, which also reacts rapidly with neutral sodium sulphite.
Characteristic Features It is a colourless liquid having a pleasant melissa like odour. It has the following physical characteristic features. bp1 47oC; bp760 203-204oC; n20D 1.4460; [α]25D + 11.50°; d= 0.848-0.856; It is very slightly soluble in water but readily soluble in alcohols.
Under improper storage conditions it slowly undergoes decomposition, polymerization and resinification.
Under direct sun-light it yields a complex mixture which consists of acetone, β-methyl adipic acid, isopulegol and menthone.
-------------------------------------------------------
* Tiemann, Ber, 32, 834 (1899).
** Gildemeister Hoffmann die Aetherischen Oele vol IV, 307-356 (4th ed. 1956)
Strong alkalies, such as: NaOH and KOH, usually resinifies citronellal. Therefore, it is always preferred to make use of relatively weaker alkalies, for instance: Na2CO3, K2CO3, for its regeneration from its corresponding bisulphite adduct.
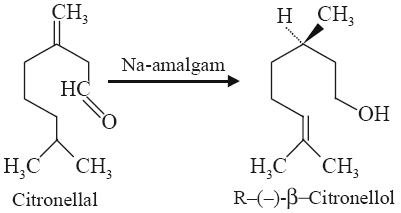
Reduction of citronellal with sodium amalgam yields citronellol i.e., a terpene alcohol, due to catalytic hydrogenation as shown below:
Identification
1. Its semicarbazone derivative has mp 91-92oC.
2. Its dinitrophenyl hydrazone derivative has mp 76.5oC.
Uses
1. For the manufacture of citronellal used in perfumery.
2. It is used largely in soap perfumes and as insect repllant.
3. It is employed as artificial citrus flavour.
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar










0 Comment:
Post a Comment